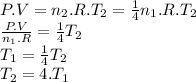Chemistry, 26.11.2019 06:31 kinziemadison12
Achemist prepares a sample of helium gas at a certain pressure, temperature and volume and then removes all but a fourth of the gas molecules (only a fourth remain). how must the temperature be changed (as a multiple of t1) to keep the pressure and the volume the same? a. t2=1/16t1b. t2=2t1c. t2=16t1d. t2= 1/2t1e. t2=4t1f. none of theseg. t2=1/4t1

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1


Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
Achemist prepares a sample of helium gas at a certain pressure, temperature and volume and then remo...
Questions



Engineering, 19.11.2020 20:10




Mathematics, 19.11.2020 20:10

Mathematics, 19.11.2020 20:10

Advanced Placement (AP), 19.11.2020 20:10


Mathematics, 19.11.2020 20:10



Health, 19.11.2020 20:10

SAT, 19.11.2020 20:10

Mathematics, 19.11.2020 20:10


Social Studies, 19.11.2020 20:10


History, 19.11.2020 20:10