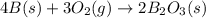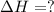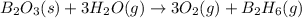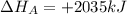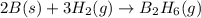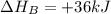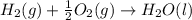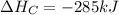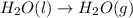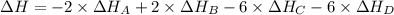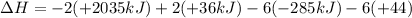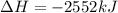Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
Calculate the enthalpy of the reaction
4b(s)+3o2(g)→2b2o3(s)
given the following pertine...
4b(s)+3o2(g)→2b2o3(s)
given the following pertine...
Questions

Mathematics, 04.06.2021 18:50

Mathematics, 04.06.2021 18:50

History, 04.06.2021 18:50


Mathematics, 04.06.2021 18:50




Mathematics, 04.06.2021 18:50

Mathematics, 04.06.2021 18:50

Mathematics, 04.06.2021 18:50

Mathematics, 04.06.2021 19:00


Mathematics, 04.06.2021 19:00

Mathematics, 04.06.2021 19:00


Mathematics, 04.06.2021 19:00


Mathematics, 04.06.2021 19:00