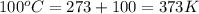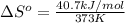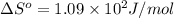Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
For water ∆h°vap = 40.7 kj/mol at 100.°c, its boiling point. calculate ∆s° for the vaporization of 1...
Questions

Chemistry, 30.08.2021 06:20


History, 30.08.2021 06:20


Health, 30.08.2021 06:20

Spanish, 30.08.2021 06:20

English, 30.08.2021 06:20

Mathematics, 30.08.2021 06:20

Mathematics, 30.08.2021 06:20


Social Studies, 30.08.2021 06:20

Mathematics, 30.08.2021 06:20

Health, 30.08.2021 06:20


Mathematics, 30.08.2021 06:20




Spanish, 30.08.2021 06:20

Mathematics, 30.08.2021 06:20

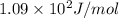
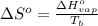
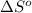 = change in entropy of vaporization = ?
= change in entropy of vaporization = ?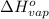 = change in enthalpy of vaporization = 40.7 kJ/mol
= change in enthalpy of vaporization = 40.7 kJ/mol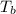 = boiling point temperature of water =
= boiling point temperature of water =