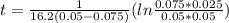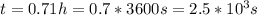a second- order reaction of the type a + b --> p was carried out in a solution that was initially 0.075 mol dm^-3 in a and 0.050 mol dm^-3 in b. after 1.0 h the concentration of a had fallen to 0.020 mol dm^-3. a) calculate the rate constant. b) solve for the half- life of each of the reactants.
hint: answers are a) 16.2 dm^3/mol*h
b) 5.1 × 10^3 s, 2.1 × 10^3 s

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 11:00
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced.be sure your answer has the correct number of significant digits.
Answers: 2
You know the right answer?
a second- order reaction of the type a + b --> p was carried out in a solution that was initially...
Questions





Biology, 25.06.2019 17:30

History, 25.06.2019 17:30


Mathematics, 25.06.2019 17:30

History, 25.06.2019 17:30

Mathematics, 25.06.2019 17:30




Mathematics, 25.06.2019 17:30

Mathematics, 25.06.2019 17:30

Mathematics, 25.06.2019 17:30


Mathematics, 25.06.2019 17:30


History, 25.06.2019 17:40

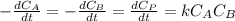
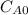 and
and 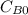 are the inital concentrations and x the concentration reacted at time t, so
are the inital concentrations and x the concentration reacted at time t, so 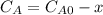 and
and 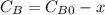 and the rate at time t is written as:
and the rate at time t is written as: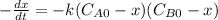
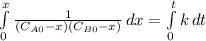
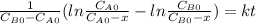
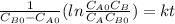
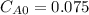 ,
, 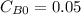 ,
,  , it implies that the quantity reacted, x, is 0.03 and
, it implies that the quantity reacted, x, is 0.03 and 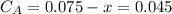 . Then, the value of k would be
. Then, the value of k would be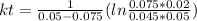
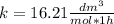
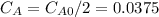 so
so 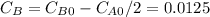 , k=16.2 and the same initial concentrations. Replacing in the equation
, k=16.2 and the same initial concentrations. Replacing in the equation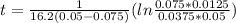
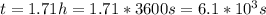
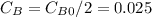 so
so 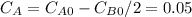 , k=16.2 and the same initial concentrations. Replacing in the equation
, k=16.2 and the same initial concentrations. Replacing in the equation