
Chemistry, 26.11.2019 07:31 malikapooh124
Calculate the percent ionization of 0.120 m lactic acid in a solution containing 8.5×10−3 m sodium lactate.
express the percent ionization to two significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3
You know the right answer?
Calculate the percent ionization of 0.120 m lactic acid in a solution containing 8.5×10−3 m sodium l...
Questions



Mathematics, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01

Arts, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01

Computers and Technology, 25.09.2020 14:01

Physics, 25.09.2020 14:01

Chemistry, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01



Computers and Technology, 25.09.2020 14:01


History, 25.09.2020 14:01

Biology, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01

Chemistry, 25.09.2020 14:01

Geography, 25.09.2020 14:01

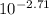 = [H⁺]
= [H⁺]

