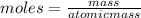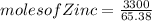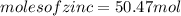Chemistry, 26.11.2019 07:31 loredohome
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant reaction is
zn2+(aq)+2e−→zn(s)
for a large batch of nails, a manufacturer needs to plate a total zinc mass of 3.30 kg on the surface to get adequate coverage.
part a
how many moles of zinc are in 3.30 kg of zinc?
express your answer to three significant figures and include the appropriate units.
50.5 mol
submithintsmy answersgive upreview part
correct
significant figures feedback: your answer 50.47mol was either rounded differently or used a different number of significant figures than required for this part.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant rea...
Questions

Mathematics, 20.06.2020 02:57







Mathematics, 20.06.2020 02:57





Biology, 20.06.2020 02:57





History, 20.06.2020 02:57

Arts, 20.06.2020 02:57