
Chemistry, 26.11.2019 19:31 dessyrob05
Which of the following is not true for an exothermic reaction?
a. the products have a higher enthalpy than reactants
b. the temperature of the surroundings rise
c. the enthalpy change for the reaction is negative heat flows from the rxn system to the surroundings

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1
You know the right answer?
Which of the following is not true for an exothermic reaction?
a. the products have a higher...
a. the products have a higher...
Questions





Mathematics, 29.01.2020 23:47


History, 29.01.2020 23:47



Health, 29.01.2020 23:47

History, 29.01.2020 23:47



Business, 29.01.2020 23:47





Social Studies, 29.01.2020 23:47

Chemistry, 29.01.2020 23:47

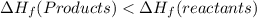 )
)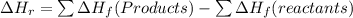
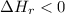 )
)

