
Chemistry, 26.11.2019 21:31 AshlynPlayz45
Consider the following reaction at constant pressure. use the information provided below to determine the value of δs at 473 k. predict whether or not this reaction will be spontaneous at this temperature.
4nh3 (g) + 3o2 (g) → 2n2 (g) + 6h2o (g) δh = –1267 kj

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1
You know the right answer?
Consider the following reaction at constant pressure. use the information provided below to determin...
Questions

History, 19.01.2021 18:40

Arts, 19.01.2021 18:40




Mathematics, 19.01.2021 18:40





Biology, 19.01.2021 18:40


History, 19.01.2021 18:40




Mathematics, 19.01.2021 18:40

Chemistry, 19.01.2021 18:40


Mathematics, 19.01.2021 18:40


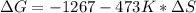 :
: ![\Delta S0[/tex}Back to this expression: [tex]\Delta G= -1267 - 473 K* \Delta S](/tpl/images/0392/0613/808f0.png)


