
Chemistry, 26.11.2019 21:31 senituliii
Sulfur has an atomic mass of 32 u and avogadro's number is 6 × 1023 formula units/mole. the number of sulfur atoms in 1 g of sulfur is:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2

Chemistry, 23.06.2019 08:50
What happens after sound waves create vibrations in the ear?
Answers: 1
You know the right answer?
Sulfur has an atomic mass of 32 u and avogadro's number is 6 × 1023 formula units/mole. the number o...
Questions


History, 06.05.2020 04:00


History, 06.05.2020 04:00

Mathematics, 06.05.2020 04:00

Mathematics, 06.05.2020 04:00


Mathematics, 06.05.2020 04:00


Mathematics, 06.05.2020 04:00

Biology, 06.05.2020 04:00



Computers and Technology, 06.05.2020 04:00




Biology, 06.05.2020 04:00

Computers and Technology, 06.05.2020 04:00

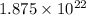 atoms
atoms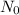 .
.
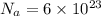
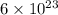 of atoms
of atoms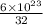 of atoms
of atoms

