
Chemistry, 26.11.2019 21:31 rebeckas0102
For the following reactions, the δh° rxn is not equal to δh° f for the product except for
a. h2o (l) + 1/2 o2 (g) → h2o2(l)
b. n2 (g) + o2 (g) → 2no (g)
c. 2h2 (g) + o2 (g) → 2h2o (g)
d. 2h2 (g) + o2 (g) → 2h2o (l)
e. 2c(s, graphite) + 2h2(g) → c2h4 (g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1
You know the right answer?
For the following reactions, the δh° rxn is not equal to δh° f for the product except for
Questions





History, 19.09.2019 19:30


Mathematics, 19.09.2019 19:30

Mathematics, 19.09.2019 19:30


Mathematics, 19.09.2019 19:30

Mathematics, 19.09.2019 19:30

Mathematics, 19.09.2019 19:30


Mathematics, 19.09.2019 19:30

History, 19.09.2019 19:30

English, 19.09.2019 19:30




Physics, 19.09.2019 19:30

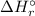 ) is the change in enthalpy associated with a given chemical reaction. It can be calculated by the standard enthalpies of formation of the products and reactants.
) is the change in enthalpy associated with a given chemical reaction. It can be calculated by the standard enthalpies of formation of the products and reactants. 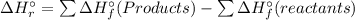
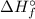 ) of an elements that is present in its standard state is zero.
) of an elements that is present in its standard state is zero.![\Delta H_{r}^{\circ} = \left [\Delta H_{f}^{\circ} (H_{2}O_{2}, l) \right ] - \left [\Delta H_{f}^{\circ} (H_{2}O, l)+ 1/2\times \Delta H_{f}^{\circ} (O_{2}, g)\right]](/tpl/images/0392/0323/5735d.png)
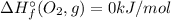
![\Delta H_{r}^{\circ} = \left [\Delta H_{f}^{\circ} (H_{2}O_{2}, l) \right ] - \left [\Delta H_{f}^{\circ} (H_{2}O, l)\right] \neq \Delta H_{f}^{\circ} (Products)](/tpl/images/0392/0323/60595.png)
![\Delta H_{r}^{\circ} = \left [2\times \Delta H_{f}^{\circ} (NO, g) \right ] - \left [\Delta H_{f}^{\circ} (N_{2}, g)+\Delta H_{f}^{\circ} (O_{2}, g)\right]](/tpl/images/0392/0323/c62af.png)
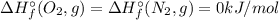
![\Delta H_{r}^{\circ} = \left [2\times \Delta H_{f}^{\circ} (NO, g) \right ] \neq \Delta H_{f}^{\circ} (Product, NO)](/tpl/images/0392/0323/39174.png)
![\Delta H_{r}^{\circ} = \left [2\times \Delta H_{f}^{\circ} (H_{2}O, g) \right ] - \left [2\times \Delta H_{f}^{\circ} (H_{2}, g)+\Delta H_{f}^{\circ} (O_{2}, g)\right]](/tpl/images/0392/0323/6e78e.png)
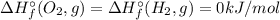
![\Delta H_{r}^{\circ} = \left [2\times \Delta H_{f}^{\circ} (H_{2}O, g) \right ] \neq \Delta H_{f}^{\circ} (Product, H_{2}O)](/tpl/images/0392/0323/b5284.png)
![\Delta H_{r}^{\circ} = \left [2\times \Delta H_{f}^{\circ} (H_{2}O, l) \right ] - \left [2\times \Delta H_{f}^{\circ} (H_{2}, g)+\Delta H_{f}^{\circ} (O_{2}, g)\right]](/tpl/images/0392/0323/a373f.png)
![\Delta H_{r}^{\circ} = \left [2\times \Delta H_{f}^{\circ} (H_{2}O, l) \right ] \neq \Delta H_{f}^{\circ} (Product, H_{2}O)](/tpl/images/0392/0323/02a0f.png)
![\Delta H_{r}^{\circ} = \left [\Delta H_{f}^{\circ} (C_{2}H_{4}, g) \right ] - \left [2\times \Delta H_{f}^{\circ} (C, s graphite)+2\times \Delta H_{f}^{\circ} (H_{2}, g)\right]](/tpl/images/0392/0323/d87f3.png)
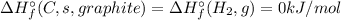
![\Delta H_{r}^{\circ} = \left [\Delta H_{f}^{\circ} (C_{2}H_{4}, g) \right ] = \Delta H_{f}^{\circ} (Product, C_{2}H_{4})](/tpl/images/0392/0323/5f4af.png)


