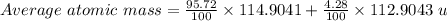Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
An element consists of two isotopes. the abundance of one isotope is 95.72% and its atomic mass is 1...
Questions

Mathematics, 10.04.2020 16:29



Mathematics, 10.04.2020 16:29


Mathematics, 10.04.2020 16:29


Mathematics, 10.04.2020 16:29

Mathematics, 10.04.2020 16:29

Mathematics, 10.04.2020 16:29










English, 10.04.2020 16:29