
Chemistry, 26.11.2019 23:31 sarahlearn3
Lithium and fluorine undergo ionic bonding. using the noble gas electron configurations for each (below), explain the process of bonding step by step, using proper grammar and mechanics. noble gas electron configurations: lithium: [he] 2s1 fluorine: [he] 2s22p5

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

You know the right answer?
Lithium and fluorine undergo ionic bonding. using the noble gas electron configurations for each (be...
Questions


Mathematics, 19.02.2021 18:20

Chemistry, 19.02.2021 18:20

Mathematics, 19.02.2021 18:20

Mathematics, 19.02.2021 18:20

Biology, 19.02.2021 18:20



Mathematics, 19.02.2021 18:20

Mathematics, 19.02.2021 18:20

Mathematics, 19.02.2021 18:20



Mathematics, 19.02.2021 18:20


History, 19.02.2021 18:20

Mathematics, 19.02.2021 18:20

Chemistry, 19.02.2021 18:20

Mathematics, 19.02.2021 18:20

Social Studies, 19.02.2021 18:20

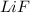 .
.![[He]2s^1](/tpl/images/0392/2650/6e198.png) .
.
![[He]2s^22p^5](/tpl/images/0392/2650/da8fe.png) .
.


