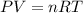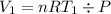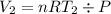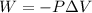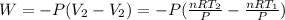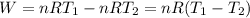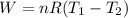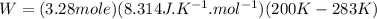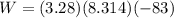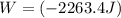Chemistry, 26.11.2019 23:31 parkerwallace04
Acylinder with a moveable piston contains 92g of nitrogen. the external pressure is constant at 1.00 atm. the initial temperature is 200k. when the temperature is increased by 83 k, by taking it out of the freezer, the volume will increase, according to the ideal gas law. calculate the work for this process. express your answer in j.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
Acylinder with a moveable piston contains 92g of nitrogen. the external pressure is constant at 1.00...
Questions

History, 03.02.2022 08:20

Mathematics, 03.02.2022 08:30




History, 03.02.2022 08:30


Law, 03.02.2022 08:30

Social Studies, 03.02.2022 08:30

Medicine, 03.02.2022 08:30


Mathematics, 03.02.2022 08:30

Mathematics, 03.02.2022 08:30

SAT, 03.02.2022 08:30

Mathematics, 03.02.2022 08:30

Mathematics, 03.02.2022 08:30



Chemistry, 03.02.2022 08:30