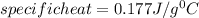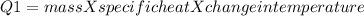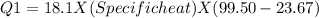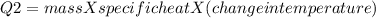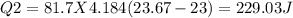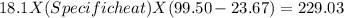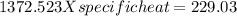Chemistry, 27.11.2019 01:31 sheram2010
In the laboratory, a student uses a coffee cup calorimeter to determine the specific heat of a metal. she heats 18.1 grams of lead to 99.50°c and then drops it into a cup containing 81.7 grams of water at 23.00°c. she measures the final temperature to be 23.67°c. assuming that all of the heat is transferred to the water, she calculates the specific heat of lead to be j/g°c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1

Chemistry, 23.06.2019 08:30
Of the following elements, which is the least reactive? a. c b. h c. li d. he
Answers: 1
You know the right answer?
In the laboratory, a student uses a coffee cup calorimeter to determine the specific heat of a metal...
Questions

Mathematics, 10.10.2020 14:01





Mathematics, 10.10.2020 14:01



Social Studies, 10.10.2020 14:01





English, 10.10.2020 14:01






Mathematics, 10.10.2020 14:01