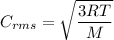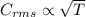Chemistry, 27.11.2019 01:31 dan20012001
If the absolute temperature of a gas is tripled, what happens to the root‑mean‑square speed of the molecules?
a. nothing happens to the rms speed.
b. the new rms speed is 9 times the original rms speed.
c. the new rms speed is 3 times the original rms speed.
d. the new rms speed is 1.732 times the original rms speed.
e. the new rms speed is 1/3 the original rms speed.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
If the absolute temperature of a gas is tripled, what happens to the root‑mean‑square speed of the m...
Questions

History, 11.10.2019 01:30

Mathematics, 11.10.2019 01:30

Spanish, 11.10.2019 01:30


History, 11.10.2019 01:30

Mathematics, 11.10.2019 01:30


Mathematics, 11.10.2019 01:30


English, 11.10.2019 01:30



Mathematics, 11.10.2019 01:30

History, 11.10.2019 01:30


Mathematics, 11.10.2019 01:30

Mathematics, 11.10.2019 01:30


History, 11.10.2019 01:30