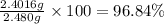Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.480 g and then makes several scratches in the copper coating (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s)+2hcl(aq)→h2(g)+zncl2(aq) the student collects the hydrogen produced over water at 25 ∘c. the collected gas occupies a volume of 0.903 l at a total pressure of 785 mmhg .
calculate the percent zinc in the penny. (assume that all the zn in the penny dissolves.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1


Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1

Chemistry, 23.06.2019 02:50
For questions 1 and 2, consider the following experimental data.hydrogen emission lines were detected at the following wavelengths (in nm): 121.6102.697.395.093.8question 1use the electromagnetic radiation classifications below and figure 1-1 in the introductory information for this lab (in the lab manual) to determine the nf value for the experimental data provided? wavelength, ? (nm) 650 700 550 600 400 450 500 visible spectrum wavelength, ? (m) 11 10 3 10 10 10 8 10 5 10 10 -10 10 9 10 10 10 10 -12 10 microwave radio infrared x-ray ultraviolet gamma 1020 1019 1018 1 1016 015 1014 01 12 109108 frequency, v (hz)a.1b. 2c. 3d. 4e. 5question 2using the data for the emission line with the longest wavelength, the known value of nf (from question 1 in this prelab), and the value of ni (deduced from the ? and nf values) calculate the rydberg constant for hydrogen (rh) in units of m-1.a) 1.097 x 10-11 m-1b) 5.921 x 107 m-1c) 1.097 x 10-2 m-1d) 9.252 x 106 m-1e) 1.097 x 107 m-1
Answers: 3
You know the right answer?
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to...
Questions








Mathematics, 16.04.2020 05:00

Mathematics, 16.04.2020 05:00






Mathematics, 16.04.2020 05:00



Mathematics, 16.04.2020 05:01


Mathematics, 16.04.2020 05:01

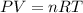 (ideal gas equation )
(ideal gas equation )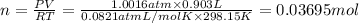
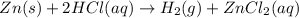
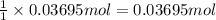 of zinc
of zinc