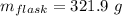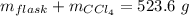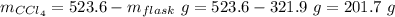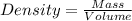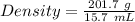Chemistry, 27.11.2019 02:31 Jakyramason
Aflask with a mass of 321.9 g is filled with 15.7 ml of carbon tetrachloride. the mass of the flask and carbon tetrachloride is found to be 523.6 g. from this information, calculate the density of carbon tetrachloride. according to this problem, the density of ccl4 is answer g/ml.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
Aflask with a mass of 321.9 g is filled with 15.7 ml of carbon tetrachloride. the mass of the flask...
Questions


Mathematics, 24.08.2019 12:30

Mathematics, 24.08.2019 12:30

Mathematics, 24.08.2019 12:30



History, 24.08.2019 12:30

Mathematics, 24.08.2019 12:30




History, 24.08.2019 12:30

Advanced Placement (AP), 24.08.2019 12:30


History, 24.08.2019 12:30


Mathematics, 24.08.2019 12:30

Chemistry, 24.08.2019 12:30


Mathematics, 24.08.2019 12:30