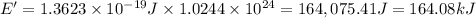Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
Determine the energy of 2.00 mol of photons for each of the following kinds of light. (assume three...
Questions

Biology, 27.09.2019 05:30


Mathematics, 27.09.2019 05:30


Mathematics, 27.09.2019 05:30


Mathematics, 27.09.2019 05:30





Mathematics, 27.09.2019 05:30


English, 27.09.2019 05:30


Mathematics, 27.09.2019 05:30


Mathematics, 27.09.2019 05:30

History, 27.09.2019 05:30

Mathematics, 27.09.2019 05:30

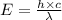
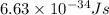
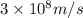
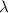 = wavelength =
= wavelength = 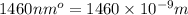
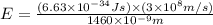
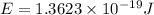
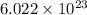 particles
particles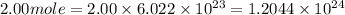 photons
photons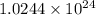 photons = E'
photons = E'