
Chemistry, 27.11.2019 02:31 lovemichelle638
Exactly 15.0 ml of an unknown acid was measured in a 25 ml graduated cylinder and poured into a 250 ml flask. the graduated cylinder was then rinsed with 25 ml of distilled water. the rinse was added to the 250 ml flask. an additional 75 ml of water was added to the flask along with 4-6 drops of the phenolphthalein indicator. the solution was then titrated with 0.288 m naoh. the initial burette reading was 1.63 ml. after titration, the final burette reading was 41.17 ml. calculate the molarity of the unknown acid. (hint: the acid base ratio is 1: 1) ha + naoh → naa + h2o

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
You know the right answer?
Exactly 15.0 ml of an unknown acid was measured in a 25 ml graduated cylinder and poured into a 250...
Questions

Chemistry, 04.02.2020 11:46

Mathematics, 04.02.2020 11:46


History, 04.02.2020 11:46


Social Studies, 04.02.2020 11:46


English, 04.02.2020 11:46

Arts, 04.02.2020 11:46

History, 04.02.2020 11:46

Mathematics, 04.02.2020 11:46

History, 04.02.2020 11:46


Mathematics, 04.02.2020 11:46






Mathematics, 04.02.2020 11:46

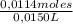 = 0,76M
= 0,76M

