
Chemistry, 27.11.2019 02:31 csciahetano
Astudent dissolved 4.00 g of co(no3)2 in enough water to make 100. ml of stock solution. he took 4.00 ml of the stock solution and then diluted it with water to give 275. ml of a final solution. how many grams of no3- ion are there in the final solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

You know the right answer?
Astudent dissolved 4.00 g of co(no3)2 in enough water to make 100. ml of stock solution. he took 4.0...
Questions

Business, 03.08.2019 07:00

Biology, 03.08.2019 07:00


History, 03.08.2019 07:00








History, 03.08.2019 07:00

Mathematics, 03.08.2019 07:00



Mathematics, 03.08.2019 07:00


Health, 03.08.2019 07:00

History, 03.08.2019 07:00

English, 03.08.2019 07:00

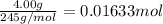
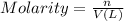
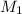
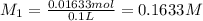
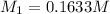
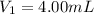
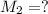 (molarity after dilution)
(molarity after dilution)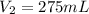 (after dilution)
(after dilution)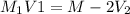
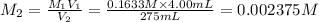
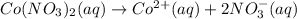
![[NO_3^{-}]=\frac{2}{1}\times 0.002375 M=0.004750 M](/tpl/images/0392/5295/92789.png)
![[NO_3^{-}]=0.004750 M](/tpl/images/0392/5295/3871f.png)
![[NO_3^{-}]=\frac{n}{0.275 L}](/tpl/images/0392/5295/06d68.png)


