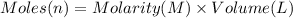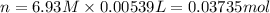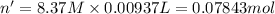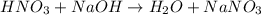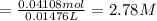Calculate the concentration of acid(or base) remaining in solution when 5.39ml of 6.93m hno3 is added to 9.37ml of 8.37m naoh. note the final volume is the sum of two added volumes. which of the following statement is true for the solution after mixing? a) naoh is in excess overhno3b)hno3 is in excess over naohc)hno3 and naoh are exactly balanced. what is the concentration of the excess naoh (or hno3) you indicated above?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2


Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
Calculate the concentration of acid(or base) remaining in solution when 5.39ml of 6.93m hno3 is adde...
Questions


Arts, 30.03.2020 23:15


Chemistry, 30.03.2020 23:15

Biology, 30.03.2020 23:15

History, 30.03.2020 23:15

Mathematics, 30.03.2020 23:15



Computers and Technology, 30.03.2020 23:15

Computers and Technology, 30.03.2020 23:15

Mathematics, 30.03.2020 23:15

Biology, 30.03.2020 23:15





Mathematics, 30.03.2020 23:16


 .
.