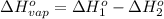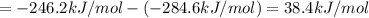Chemistry, 27.11.2019 03:31 KariSupreme
At a given set of conditions, 246.2 kj is given off when 1 mol of h2o(g) forms from its elements. under the same conditions, 284.6 kj is given off when 1 mol of h2o(l) forms from its elements. find δh for the vaporization of water at these conditions.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
At a given set of conditions, 246.2 kj is given off when 1 mol of h2o(g) forms from its elements. un...
Questions

Mathematics, 05.05.2020 11:30

English, 05.05.2020 11:30

Mathematics, 05.05.2020 11:30

Mathematics, 05.05.2020 11:30


History, 05.05.2020 11:30

Mathematics, 05.05.2020 11:30

Chemistry, 05.05.2020 11:30


Mathematics, 05.05.2020 11:30


English, 05.05.2020 11:30

Mathematics, 05.05.2020 11:30


Chemistry, 05.05.2020 11:30

Mathematics, 05.05.2020 11:30


Mathematics, 05.05.2020 11:30

Chemistry, 05.05.2020 11:30

Mathematics, 05.05.2020 11:30

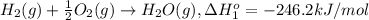 ..[1]
..[1]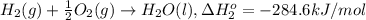 ..[2]
..[2]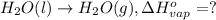 ...[3]
...[3]