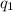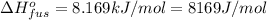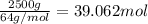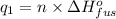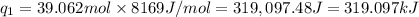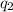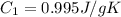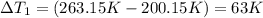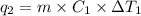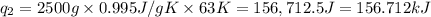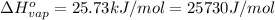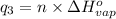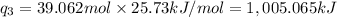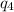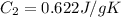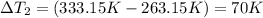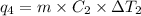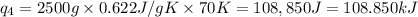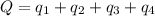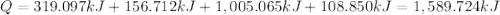Sulfur dioxide is produced in enormous amounts for sulfuric acid production. it melts at - 73˚c and boils at - 10˚c. its δh˚fus is 8.619 kj/mol and its δh˚vap is 25.73 kj/mol. the specific heat capacities of the liquid and the gas are 0.995 j/g·k and 0.622 j/g·k, respectively. how much heat is required to convert 2.500 kg of solid so₂ at the melting point to a gas at 60˚c?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1


Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Sulfur dioxide is produced in enormous amounts for sulfuric acid production. it melts at - 73˚c and...
Questions








Geography, 26.06.2019 12:10

Geography, 26.06.2019 12:10


Mathematics, 26.06.2019 12:10

Biology, 26.06.2019 12:10

Physics, 26.06.2019 12:10

Mathematics, 26.06.2019 12:10



Biology, 26.06.2019 12:10

Chemistry, 26.06.2019 12:10