
Chemistry, 27.11.2019 04:31 johnny2585
What is the theoretical yield of vanadium, in moles, that can be produced by the reaction of 1.0 mole of v2o5 with 4.0 moles of calcium based on the following chemical equation? v2o5(s) + 5ca(l) → 2v(l) + 5cao(s)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
What is the theoretical yield of vanadium, in moles, that can be produced by the reaction of 1.0 mol...
Questions




Advanced Placement (AP), 25.11.2019 01:31


History, 25.11.2019 01:31

Mathematics, 25.11.2019 01:31






Mathematics, 25.11.2019 01:31


Mathematics, 25.11.2019 01:31


Mathematics, 25.11.2019 01:31



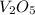 = 1.0 moles
= 1.0 moles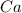 = 4.0 moles
= 4.0 moles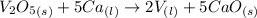
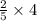 mole of V
mole of V


