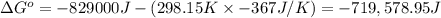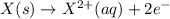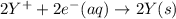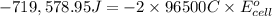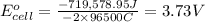Chemistry, 27.11.2019 04:31 rostecorralmart
Calculate the standard cell potential at 25 ∘c for the reactionx(s)+2y+(aq)→x2+(aq)+2y(s)w here δh∘ = -829 kj and δs∘ = -367 j/k

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 01:00
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1
You know the right answer?
Calculate the standard cell potential at 25 ∘c for the reactionx(s)+2y+(aq)→x2+(aq)+2y(s)w here δh∘...
Questions




Mathematics, 26.06.2019 14:10

Mathematics, 26.06.2019 14:10

Mathematics, 26.06.2019 14:10


Chemistry, 26.06.2019 14:10

Business, 26.06.2019 14:10


Mathematics, 26.06.2019 14:10


Chemistry, 26.06.2019 14:10

Chemistry, 26.06.2019 14:10



Mathematics, 26.06.2019 14:10

History, 26.06.2019 14:10


Mathematics, 26.06.2019 14:10


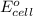 = standard electrode potential of the cell
= standard electrode potential of the cell