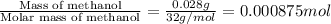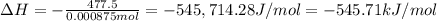Abomb calorimetric experiment was run to determine the enthalpy of combustion of methanol. the reaction is ch3oh(l)+3/2o2(g)→co2(g)+2h2o(l) the bomb calorimeter has a heat capacity of 250.0 j/k. burning 0.028 g of methanol resulted in a rise in temperature from 21.50 ∘c to 23.41 ∘c. calculate the change in internal energy for the combustion of methanol in kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
Abomb calorimetric experiment was run to determine the enthalpy of combustion of methanol. the react...
Questions


Chemistry, 15.10.2020 02:01



Mathematics, 15.10.2020 02:01




Mathematics, 15.10.2020 02:01

Mathematics, 15.10.2020 02:01

Mathematics, 15.10.2020 02:01

Mathematics, 15.10.2020 02:01




Mathematics, 15.10.2020 02:01


Mathematics, 15.10.2020 02:01


English, 15.10.2020 02:01

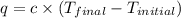
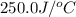
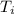 = Initial temperature =
= Initial temperature = 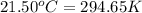
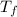 = Final temperature =
= Final temperature = 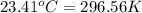
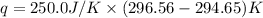
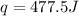
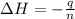
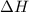 = enthalpy change = ?
= enthalpy change = ?