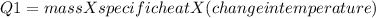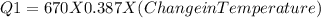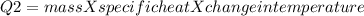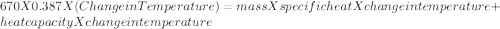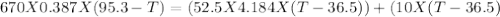Chemistry, 27.11.2019 06:31 eylinglez3ovm16v
A670.-g piece of copper tubing is heated to 95.3°c and placed in an insulated vessel containing 52.5 g of water at 36.5°c. assuming no loss of water and heat capacity of 10.0 j/k for the vessel, what is the final temperature (c of copper = 0.387 j/g · k)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1


Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2
You know the right answer?
A670.-g piece of copper tubing is heated to 95.3°c and placed in an insulated vessel containing 52.5...
Questions

English, 08.11.2020 19:40


Mathematics, 08.11.2020 19:40


Social Studies, 08.11.2020 19:40


Mathematics, 08.11.2020 19:40

Mathematics, 08.11.2020 19:40

English, 08.11.2020 19:40




Mathematics, 08.11.2020 19:40



Mathematics, 08.11.2020 19:40



Mathematics, 08.11.2020 19:40