
Chemistry, 27.11.2019 06:31 mdaniella522
2-phosphoglycerate(2pg) is converted to phosphoenolpyruvate (pep) by the enzyme enolase. the standard free energy change(deltago’) for this reaction is +1.7 kj/mol. if the cellular concentrations are 2pg = 0.5 mm and pep = 0.1 mm, what is the free energy change at 37 oc for the reaction 2pg ↔ pep? (a) 5.8 kj/mol(b) -5.8 kj/mol(c) +2.4 kj/mol(d) -2.4 kj/mol(e) -4146.4 kj/mol(f) +4146.4 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2


Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
2-phosphoglycerate(2pg) is converted to phosphoenolpyruvate (pep) by the enzyme enolase. the standar...
Questions




History, 25.02.2020 19:48


English, 25.02.2020 19:48














Arts, 25.02.2020 19:48

![Q_{r} =\frac{\left [ PEP \right ]}{\left [ 2PG \right ]} = \frac{0.1 mM}{0.5 mM} = 0.2](/tpl/images/0392/9257/2f0c6.png)
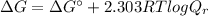
![\Delta G = 1.7 kJ/mol + [2.303 \times (8.314 \times 10^{-3} kJ/(K.mol))\times (310.15 K)] log (0.2)](/tpl/images/0392/9257/2a926.png)
![\Delta G = 1.7 + [5.938] \times (-0.699) = 1.7 - 4.15 = (-2.45 kJ/mol)](/tpl/images/0392/9257/65b39.png)


