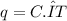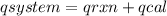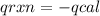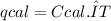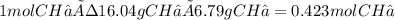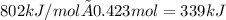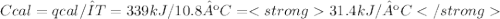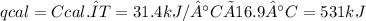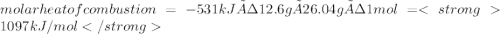Chemistry, 27.11.2019 07:31 stophendless9780
The heat capacity of a bomb calorimeter was determined by burning 6.79 g methane (energy of combustion 802 kj/mol ch4) in the bomb. the temperature changed by 10.8c. a. what is the heat capacity of the bomb? b. a 12.6-g sample of acetylene, c2h2, produced a temperature increase of 16.9c in the same calorimeter. what is the energy of combustion of acetylene (in kj/mol)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
The heat capacity of a bomb calorimeter was determined by burning 6.79 g methane (energy of combusti...
Questions




Chemistry, 31.03.2020 04:20





Mathematics, 31.03.2020 04:20


Chemistry, 31.03.2020 04:20

Mathematics, 31.03.2020 04:20




Mathematics, 31.03.2020 04:20