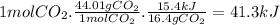Chemistry, 27.11.2019 19:31 eheheh80ii
The reaction of carbon dioxide(g) with hydrogen(g) to form carbon monoxide(g) and water(g) proceeds as follows: co2(g) + h2(g) > co(g) + h2o(g)when 16.4 grams of co2(g) react with sufficient h2(g) , 15.4 kj of energy areabsorbed .what is the value of > h for the chemical equation given? δhrxn = kj

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3
You know the right answer?
The reaction of carbon dioxide(g) with hydrogen(g) to form carbon monoxide(g) and water(g) proceeds...
Questions