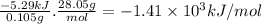Chemistry, 27.11.2019 19:31 billyeyelash
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was burned in this calorimeter, the temperature increased by 2.14 k. calculate the energy of combustion for one mole of ethylene. a. –1.41 × 103 kj/mol b. –660 kj/mol c. –5.29 kj/mol d. –0.259 kj/mol e. –50.3 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was bur...
Questions



English, 01.12.2020 18:00

Mathematics, 01.12.2020 18:00


Mathematics, 01.12.2020 18:00

History, 01.12.2020 18:00



Mathematics, 01.12.2020 18:00





Mathematics, 01.12.2020 18:00



Mathematics, 01.12.2020 18:00


Mathematics, 01.12.2020 18:00