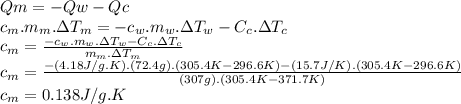Chemistry, 27.11.2019 20:31 cindyc1103
307 g of an unknown mineral is heated to 98.7 °c and placed into a calorimeter that contains 72.4 g of water at 23.6 °c. the heat capacity of the empty calorimeter was 15.7 j/k. the final temperature of the calorimeter was 32.4 °c. what is the specific heat capacity of the mineral in j/gk?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
307 g of an unknown mineral is heated to 98.7 °c and placed into a calorimeter that contains 72.4 g...
Questions

Physics, 25.01.2020 06:31