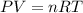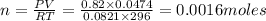Chemistry, 27.11.2019 21:31 robert7248
In an electrolysis experiment such as the one you are going to perform, 47.4 ml of hydrogen gas were collected. the temperature was 23 degrees celsius and the barometric pressure was 643 mm hg. the vapor pressure of water at 23 degrees celsius is 21 mm hg. the number of moles of hydrogen gas formed in the experiment was

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3

Chemistry, 23.06.2019 10:30
Ethyl alcohol, also known as ethanol, has a density of 0.79 g/ml. what is the volume, in quarts, of 1.95 kg of this alcohol?
Answers: 2
You know the right answer?
In an electrolysis experiment such as the one you are going to perform, 47.4 ml of hydrogen gas were...
Questions



English, 11.05.2021 19:10

Mathematics, 11.05.2021 19:10




Mathematics, 11.05.2021 19:10

Computers and Technology, 11.05.2021 19:10

Computers and Technology, 11.05.2021 19:10

English, 11.05.2021 19:10

Mathematics, 11.05.2021 19:10



Mathematics, 11.05.2021 19:10

Mathematics, 11.05.2021 19:10




Chemistry, 11.05.2021 19:10