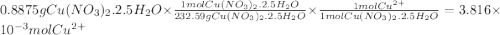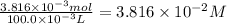Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 12:30
Idid a lab for chemistry where we put nails in a copper (ii) chloride solution. 1. why did the reaction stop? which reactant was used up? how do you know? 2. describe what was happening to the atoms of iron and copper during the reaction. what is this type of reaction called? 3. what would happen to the ratio of copper to iron if you had placed more nails in the beaker? if you had let the reaction go for less time? 4. what is the accepted ratio of copper atoms to iron atoms in this reaction? account for differences between your experimental value and the accepted value. write the balanced equation for the reaction.
Answers: 2
You know the right answer?
Astock solution of cu2+(aq) was prepared by placing 0.8875 g of solid cu(no3)2∙2.5 h2o in a 100.0-ml...
Questions


Mathematics, 30.11.2021 22:10

Mathematics, 30.11.2021 22:10




Mathematics, 30.11.2021 22:10




Mathematics, 30.11.2021 22:10

Biology, 30.11.2021 22:10


Social Studies, 30.11.2021 22:10

Social Studies, 30.11.2021 22:10

Chemistry, 30.11.2021 22:10



Mathematics, 30.11.2021 22:10

English, 30.11.2021 22:10