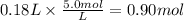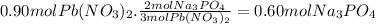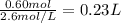Chemistry, 27.11.2019 22:31 marelinatalia2000
Assume 0.18 l of a 5.0 m solution of lead (ii) nitrate, pb(no3)2, reacts with a 2.6 m solution of sodium phosphate, na3po4, to produce lead (ii) phosphate, pb3(po4)2, and sodium nitrate, nano3. the problem requires that you determine the volume of sodium phosphate, na3po4, needed for the reaction to occur.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3
You know the right answer?
Assume 0.18 l of a 5.0 m solution of lead (ii) nitrate, pb(no3)2, reacts with a 2.6 m solution of so...
Questions



Mathematics, 29.10.2020 19:50


Health, 29.10.2020 19:50

Biology, 29.10.2020 19:50

Mathematics, 29.10.2020 19:50

Mathematics, 29.10.2020 19:50


Health, 29.10.2020 19:50

Mathematics, 29.10.2020 19:50


Mathematics, 29.10.2020 19:50



Arts, 29.10.2020 19:50



Mathematics, 29.10.2020 19:50

Mathematics, 29.10.2020 19:50