Chemistry, 27.11.2019 22:31 wardlawshaliyah
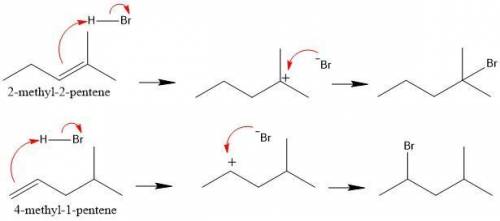
Identify which of the following two reactions you would expect to occur more rapidly: (1) addition of hbr to 2-methyl-2-pentene or (2) addition of hbr to 4-methyl-1-pentene. explain your choice. addition of hbr to should be more rapid because the reaction can proceed via a carbocation. in contrast, addition of hbr to proceeds via a less stable, carbocation.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2

Chemistry, 23.06.2019 11:30
Which of these have the same number of particles as 1 mole of water h2o
Answers: 1
You know the right answer?
Identify which of the following two reactions you would expect to occur more rapidly: (1) addition...
Questions

Mathematics, 16.12.2021 08:50




Mathematics, 16.12.2021 08:50

Mathematics, 16.12.2021 08:50


Mathematics, 16.12.2021 08:50


English, 16.12.2021 08:50

Mathematics, 16.12.2021 08:50





Mathematics, 16.12.2021 08:50

English, 16.12.2021 08:50


Mathematics, 16.12.2021 08:50