Chemistry, 27.11.2019 22:31 harleypage308
Suppose 1.00 mol superheated ice melts to liquid water at 25°c. assume the specific heats of ice and liquid water have the same value and are independent of temperature. the enthalpy change for the melting of ice at 0°c is 6007 j mol21. calculate dh, dssys, and dg for this process.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

You know the right answer?
Suppose 1.00 mol superheated ice melts to liquid water at 25°c. assume the specific heats of ice and...
Questions

History, 28.06.2019 06:30



Mathematics, 28.06.2019 06:30



History, 28.06.2019 06:30


Mathematics, 28.06.2019 06:30




Mathematics, 28.06.2019 06:30


Physics, 28.06.2019 06:30





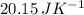
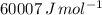
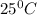
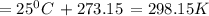
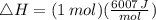
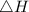 ) for the melting of 1.00 mole of ice at
) for the melting of 1.00 mole of ice at 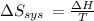
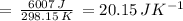
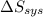 ) for the melting of 1.00 mole of ice at
) for the melting of 1.00 mole of ice at 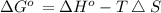
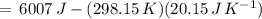
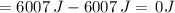
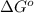 ) for the melting of 1.00 mole of ice at
) for the melting of 1.00 mole of ice at