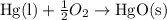Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3


You know the right answer?
When 18.5 g of hgo(s) is decomposed to form hg(l) and o2(g), 7.75 kj of heat is absorbed at standard...
Questions








Mathematics, 16.01.2020 18:31

English, 16.01.2020 18:31

Social Studies, 16.01.2020 18:31

Biology, 16.01.2020 18:31





History, 16.01.2020 18:31

Biology, 16.01.2020 18:31

Mathematics, 16.01.2020 18:31