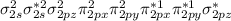Chemistry, 27.11.2019 23:31 zachthomas024
Which of the following statements relating to molecular orbital (mo) theory is incorrect? a. combination of two 2p orbitals may result in either latex: \sigma σ or latex: \pi π mos. b. combination of two atomic orbitals produces one bonding and one antibonding mo. c. a bonding mo is lower in energy than the two atomic orbitals from which it is formed. d. in a stable molecule having an even number of electrons, all electrons must be paired. e. a species with a bond order of zero will not be stable.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
Which of the following statements relating to molecular orbital (mo) theory is incorrect? a. combina...
Questions



Mathematics, 26.04.2020 06:59

Computers and Technology, 26.04.2020 06:59


Social Studies, 26.04.2020 06:59



Mathematics, 26.04.2020 06:59




Mathematics, 26.04.2020 06:59



History, 26.04.2020 06:59



English, 26.04.2020 06:59

Mathematics, 26.04.2020 06:59