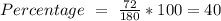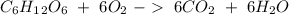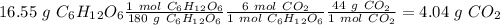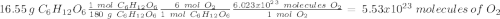Glucose (c6h12o6) is a key nutrient for generating chemical potential energy in biological systems. we were provided 16.55 g of glucose. calculate:
a) the mass percent of carbon in glucose.
b) the mass of co2 produced by the combustion of 16.55 g glucose with sufficient oxygen gas.
c) how many oxygen molecules needed for the completely combustion of 16.55 g glucose?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
Glucose (c6h12o6) is a key nutrient for generating chemical potential energy in biological systems....
Questions





Mathematics, 05.05.2021 21:30




Geography, 05.05.2021 21:30


Chemistry, 05.05.2021 21:30

Mathematics, 05.05.2021 21:30


Mathematics, 05.05.2021 21:30


Mathematics, 05.05.2021 21:30




Mathematics, 05.05.2021 21:30

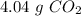
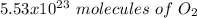
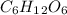 , so the first step is to find the atomic mass of each atom and multiply by the amount of atoms in the molecule.
, so the first step is to find the atomic mass of each atom and multiply by the amount of atoms in the molecule.