
Chemistry, 28.11.2019 02:31 amanquen35
Find the equilibrium partial pressures of a and b for each of the following different values of kp.? consider the following reaction: a(g) = 2b(g)find the equilibrium partial pressures of a and b for each of the following different values of kp. assume that the initial partial pressure of b in each case is 1.0 atm and that the initial partial pressure of a is 0.0 atm. make any appropriate simplifying assumptions. kp = 1.4? kp = 2.0 * 10^-4? kp = 2.0 * 10^5?

Answers: 2


Another question on Chemistry




You know the right answer?
Find the equilibrium partial pressures of a and b for each of the following different values of kp.?...
Questions

Mathematics, 04.07.2019 13:20


English, 04.07.2019 13:20

English, 04.07.2019 13:20

Mathematics, 04.07.2019 13:20


Social Studies, 04.07.2019 13:20



English, 04.07.2019 13:20


History, 04.07.2019 13:20







Social Studies, 04.07.2019 13:30

Chemistry, 04.07.2019 13:30

![P_{[A] = 0,22](/tpl/images/0394/1164/9afd7.png) ,
, ![P_{[B] = 0,56atm](/tpl/images/0394/1164/02e2b.png)
![P_{[A] = 0,495](/tpl/images/0394/1164/54750.png) ,
, ![P_{[B] = 0,01atm](/tpl/images/0394/1164/da9a1.png)
![P_{[A] = 5x10^{-6}](/tpl/images/0394/1164/9be03.png) ,
, ![P_{[B] = 0,99999atm](/tpl/images/0394/1164/07800.png)
![kp = \frac{P_{[B]}^2}{P_{[A]}}](/tpl/images/0394/1164/0f2b8.png)
![P_{[A] = 0,0atm + X](/tpl/images/0394/1164/62221.png)
![P_{[B] = 1,0atm - 2X](/tpl/images/0394/1164/dd6ae.png)
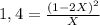
![P_{[B] = 1,0atm - 0,44atm = 0,56atm](/tpl/images/0394/1164/4f71b.png)
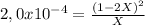
![P_{[A] = 0,495atm](/tpl/images/0394/1164/e1edd.png)
![P_{[B] = 1,0atm - 0,99atm = 0,01atm](/tpl/images/0394/1164/aeba4.png)
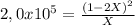
![P_{[B] = 1,0atm - 0,00001atm = 0,99999atm](/tpl/images/0394/1164/ca009.png)


