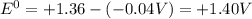Chemistry, 28.11.2019 03:31 jaylabeatty44
Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25�c. (the equation is balanced.)
3 cl2(g) + 2 fe(s) --> 6 cl-(aq) + 2 fe3+(aq)
cl2(g) + 2 e- --> 2 cl-(aq); e� = +1.36 v
fe3+(aq) + 3 e- --> fe(s); e� = -0.04 v
+1.32 v
-1.32 v
-1.40 v
+1.40 v
+4.16 v

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
Use the standard half-cell potentials listed below to calculate the standard cell potential for the...
Questions

Chemistry, 27.09.2019 08:10


Biology, 27.09.2019 08:10


Health, 27.09.2019 08:10

Mathematics, 27.09.2019 08:10




History, 27.09.2019 08:10


Social Studies, 27.09.2019 08:10

History, 27.09.2019 08:10

Health, 27.09.2019 08:10

Mathematics, 27.09.2019 08:10




Mathematics, 27.09.2019 08:10

Business, 27.09.2019 08:10

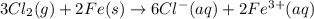
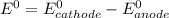
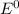 are standard reduction potentials.
are standard reduction potentials.
![E^0_{[Fe^{3+}/Fe]}=-0.04V](/tpl/images/0394/1963/07a45.png)
![E^0_{[Cl_2/Cl^-]}=+1.36V](/tpl/images/0394/1963/05c05.png)
![E^0=E^0_{[Cl_2/Cl^-]}- E^0_{[Fe^{3+}/Fe]}](/tpl/images/0394/1963/d357e.png)