
Chemistry, 28.11.2019 04:31 dbn4everloved8
A1.00 g sample of n-hexane (c6h14) undergoes complete combustion with excess o2 in a bomb calorimeter. the temperature of the 1502 g of water surrounding the bomb rises from 22.64°c to 29.30°c. the heat capacity of the hardware component of the calorimeter (everything that is not water) is 4042 j/°c. what is δu for the combustion of n-c6h14? one mole of n-c6h14 is 86.1 g. the specific heat of water is 4.184 j/g·°c.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
A1.00 g sample of n-hexane (c6h14) undergoes complete combustion with excess o2 in a bomb calorimete...
Questions


Mathematics, 26.01.2020 14:31


English, 26.01.2020 14:31



History, 26.01.2020 14:31

Mathematics, 26.01.2020 14:31



History, 26.01.2020 14:31

Mathematics, 26.01.2020 14:31


Mathematics, 26.01.2020 14:31


Health, 26.01.2020 14:31

Social Studies, 26.01.2020 14:31

English, 26.01.2020 14:31

Mathematics, 26.01.2020 14:31

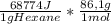 = -5,921x10⁶J/mol
= -5,921x10⁶J/mol


