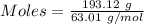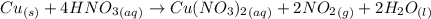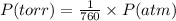Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
What volume of nitrogen dioxide is formed at 735 torr and 28.2 °c by reacting 3.56 cm3 of copper (d...
Questions




Mathematics, 21.06.2021 17:50

English, 21.06.2021 17:50

Mathematics, 21.06.2021 17:50

Chemistry, 21.06.2021 17:50


Mathematics, 21.06.2021 17:50

Biology, 21.06.2021 17:50

Mathematics, 21.06.2021 17:50

Mathematics, 21.06.2021 17:50

English, 21.06.2021 17:50

Mathematics, 21.06.2021 17:50



Mathematics, 21.06.2021 17:50

Mathematics, 21.06.2021 17:50

English, 21.06.2021 17:50

Mathematics, 21.06.2021 17:50

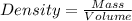
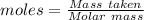
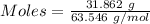
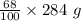 = 193.12 g
= 193.12 g