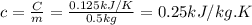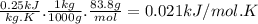Chemistry, 28.11.2019 05:31 emmazhu1106
When 18.9 kj is transferred to a gas sample in a constant volume adiabatic container with a calorimeter constant of 2.22 kj/k, the temperature of the gas (and the calorimeter) increases by 8.06 k. (a) what is the heat capacity of the sample? (b) if the sample has a mass of 0.5 kilograms, what is the specific heat capacity of the substance? (c) if the sample is krypton, what is the molar heat capacity at constant volume of krypton? the molar mass of krypton is 83.8 grams/mole.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3


Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
When 18.9 kj is transferred to a gas sample in a constant volume adiabatic container with a calorime...
Questions

Mathematics, 07.07.2019 09:30

Mathematics, 07.07.2019 09:30


Biology, 07.07.2019 09:30

Mathematics, 07.07.2019 09:30



Mathematics, 07.07.2019 09:30




Mathematics, 07.07.2019 09:30



Mathematics, 07.07.2019 09:30


Mathematics, 07.07.2019 09:30



Chemistry, 07.07.2019 09:30