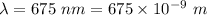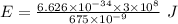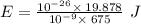Chemistry, 28.11.2019 05:31 zianebonankenotdbev
An intense emission line for a new element is observed at a wavelength of 675 nm. what is the energy of a single photon of this light? energy =

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1
You know the right answer?
An intense emission line for a new element is observed at a wavelength of 675 nm. what is the energy...
Questions

Mathematics, 01.09.2019 14:30



Social Studies, 01.09.2019 14:30


History, 01.09.2019 14:30


Mathematics, 01.09.2019 14:30

Social Studies, 01.09.2019 14:30





Mathematics, 01.09.2019 14:30

History, 01.09.2019 14:30

Chemistry, 01.09.2019 14:30

Social Studies, 01.09.2019 14:30

Mathematics, 01.09.2019 14:30

Social Studies, 01.09.2019 14:30

Mathematics, 01.09.2019 14:30

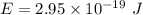
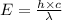
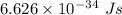
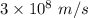
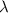 is the wavelength of the light
is the wavelength of the light