
Chemistry, 28.11.2019 06:31 bernadetteindre6650
Calculate the ph of the solution formed when 45.0 ml of 0.100m naoh solution is added to 50.0 ml of 0.100m ch3cooh (ka for acetic acid = 1.8 x10-5 ).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

You know the right answer?
Calculate the ph of the solution formed when 45.0 ml of 0.100m naoh solution is added to 50.0 ml of...
Questions


English, 05.11.2019 04:31

History, 05.11.2019 04:31


Mathematics, 05.11.2019 04:31

Mathematics, 05.11.2019 04:31

Biology, 05.11.2019 04:31

Chemistry, 05.11.2019 04:31

Mathematics, 05.11.2019 04:31




Mathematics, 05.11.2019 04:31

Mathematics, 05.11.2019 04:31




History, 05.11.2019 04:31


![pH=pKa+log(\frac{[salt]}{[acid]} )](/tpl/images/0394/4621/dd797.png)
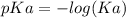
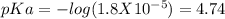
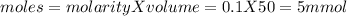
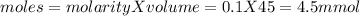
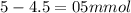
![pH=pKa+log(\frac{[salt]}{[acid]} )=4.74+log(\frac{4.5}{0.5})=5.69](/tpl/images/0394/4621/b17c8.png)


