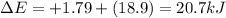Chemistry, 28.11.2019 06:31 SophieStar15
What is δe in kj for a system that receives 1.79 kj of heat from surroundings and has 4.51 kcal of work done on it at the same time. 1 cal = 4.184 j.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1

You know the right answer?
What is δe in kj for a system that receives 1.79 kj of heat from surroundings and has 4.51 kcal of w...
Questions

Health, 26.02.2020 20:28

Biology, 26.02.2020 20:28




Biology, 26.02.2020 20:28


Mathematics, 26.02.2020 20:28


Health, 26.02.2020 20:28


Mathematics, 26.02.2020 20:28




Mathematics, 26.02.2020 20:28




Chemistry, 26.02.2020 20:28

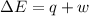
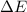 =Change in internal energy
=Change in internal energy
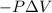 {Work is done on the system is positive as the final volume is lesser than initial volume}
{Work is done on the system is positive as the final volume is lesser than initial volume}
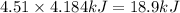 (1kcal = 4.184kJ)
(1kcal = 4.184kJ)