
Chemistry, 28.11.2019 06:31 mxltie1651
A9.000l tank at 27.0°c is filled with 5.29g of sulfur tetrafluoride gas and 15.6g of carbon dioxide gas. you can assume both gases behave as ideal gases under these conditions. calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. round each of your answers to 3 significant digits. sulfur tetraflouride: mole fraction? partial pressure? carbon dioxide: mole fraction? partial pressure? total pressure in tank?

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 10:00
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 1

Chemistry, 23.06.2019 10:30
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2
You know the right answer?
A9.000l tank at 27.0°c is filled with 5.29g of sulfur tetrafluoride gas and 15.6g of carbon dioxide...
Questions

History, 25.07.2019 13:20




English, 25.07.2019 13:20


Advanced Placement (AP), 25.07.2019 13:20


History, 25.07.2019 13:20



Mathematics, 25.07.2019 13:20

Physics, 25.07.2019 13:20

Computers and Technology, 25.07.2019 13:20




Mathematics, 25.07.2019 13:30


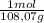 = 0,0489 moles SF₄
= 0,0489 moles SF₄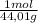 = 0,354 moles CO₂
= 0,354 moles CO₂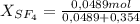 = 0,121
= 0,121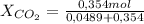 = 0,879
= 0,879

