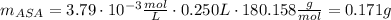Chemistry, 28.11.2019 07:31 oneicyahdaley10
(1.) using beer's law, how will the absorbance measured for the solutions change as the concentration of aspirin in solutions increase?
(2.) in an experiment, the beer’s law plot resulted in the following relationship between absorbance and concentration of asa, y = 1061.5x, where y is absorbance and x is the concentration. if the absorbance of a sample solution prepared from an aspirin tablet is 0.402, calculate the concentration of asa in the solution in m.
(3.) if the above solution was prepared by taking 10 ml of stock solution and diluting it to 100 ml, what is the concentration of the stock solution?
(4.) this stock solution was prepared as follows: an aspirin tablet was transferred to an erlenmeyer flask and reacted with naoh. the resulting solution was transferred to a 250 ml volumetric flask and the volume made up to 250 ml. calculate the mass of aspirin in the tablet based on the concentration of aspirin in the stock solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1
You know the right answer?
(1.) using beer's law, how will the absorbance measured for the solutions change as the concentratio...
Questions





World Languages, 12.09.2019 04:10



English, 12.09.2019 04:10


Mathematics, 12.09.2019 04:10


Business, 12.09.2019 04:10

Mathematics, 12.09.2019 04:10







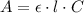 (1)
(1)![A = 1061.5 \cdot [ASA]](/tpl/images/0394/5398/223a2.png)
![[ASA] = \frac{A}{1061.5} = 3.79 \cdot 10^{-4}M](/tpl/images/0394/5398/3c100.png)
![V_{i} [ASA]_{i} = V_{f} [ASA]_{f}](/tpl/images/0394/5398/57531.png)
![[ASA]_{i} = \frac{V_{f} \cdot [ASA]_{f}}{V_{i}} = \frac {100mL \cdot 3.79\cdot 10^{-4} M}{10mL} = 3.79 \cdot 10^{-3} M](/tpl/images/0394/5398/a10be.png)
![m_{ASA} = \eta_{ASA} \cdot M_{ASA} = [ASA]_{i} \cdot V_{0} \cdot M_{ASA}](/tpl/images/0394/5398/9bfc0.png)